By Ben Leonard, Politico
Doctors have sometimes failed to diagnose serious cases of Covid-19 among people of color — and the Food and Drug Administration acknowledges one reason may be flaws in devices it approved to measure blood oxygen levels.
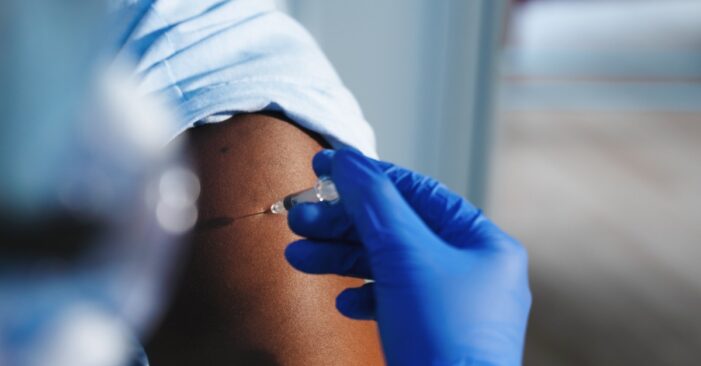
Pulse oximeters can overestimate blood oxygen in people with dark skin, causing doctors to miss patients’ distress signals.
The FDA is convening an expert advisory panel later this year to assess the problem and offer guidance to health care providers. An agency spokesperson said it has prioritized assuring that pulse oximeters are “sufficiently safe and accurate for all people.” But advocates still say the FDA, which issued a warning about the issue last year, is moving too slowly.
“It’s really shocking that it was only until 2021 for the FDA to actually issue an alert,” said Uché Blackstock, an emergency medicine physician and CEO of Advancing Health Equity. “And even in that alert last year, they didn’t even mention racial bias or race or racism in it.”
The problem raises broader concerns about bias as technology becomes more embedded in health care, and about the government’s ability to counteract it through regulation and oversight. Experts warn that disparate outcomes among racial groups could get worse if technology doesn’t work for all patients.
Researchers identified problems with pulse oximeters years ago, with small studies pointing to misreadings in people of color in 1990, 2005 and 2007.
The Covid-19 pandemic brought renewed attention to the devices, which commonly come in the form of a sensor on a patient’s fingertip.
Michael Sjoding, a pulmonary and critical care physician at the University of Michigan, conducted a study published in December 2020 in the New England Journal of Medicine that found Black patients between January and July 2020 as well as 2014 and 2015 were about three times more likely than white ones to have low blood oxygen levels go undetected. More than one in 10 Black patients with an oxygen saturation reading of 92 to 96 percent on a pulse oximeter actually had levels below 88 percent when measured by blood tests.
Normal levels range from 95 to 100 percent, while levels below 88 percent are considered dangerous.
Experts also say the problem points to the need to update guidance to compensate for the problem, as well as to diversify clinical trials.
The effect on care is real, Sjoding said. “That level of difference, had it been recognized and detected, would have changed how we would care for a patient,” he explained. “You would give a patient more oxygen or potentially give a patient different treatments.”
Known and unknown
Experts say the flawed readings are the result of how light is absorbed on different skin shades. Pulse oximeters work by shooting light onto a person’s skin and observing how much bounces back, said Achuta Kadambi, an engineering professor at the University of California, Los Angeles.
Darker skin reflects back a smaller signal than lighter skin, which can corrupt the pulse oximeter’s reading, he said. Kadambi, who has darker skin, has encountered similar problems with automated soap dispensers, which also rely on light to activate.
“The laws of physics are against darker objects, which include skin,” Kadambi said, adding that algorithms are one way to correct the issue.
But the research findings so far have limitations because they haven’t all differentiated by the type of oximeter, said Amira Mohamed, a professor at the Albert Einstein College of Medicine and a critical care specialist at Montefiore Health System. She also noted that generalizing by race can also be tricky.
“There are different types of Black people,” Mohamed said. “I’m Black myself and it doesn’t mean that it’s going to work the same way on me like it would for, for example, my husband, who’s a darker-skinned Black person.”
Mohamed also says that the existing research was conducted mostly on people with white skin and that future studies should focus on people more likely to be affected.
Potential solutions
Current FDA guidance recommends manufacturers’ studies include a minimum of 10 people and “at least 2 darkly pigmented subjects” or 15 percent of the overall group.
Some experts argue the FDA needs to make that pool larger.
“Fundamentally, you’re not going to have enough information about the accuracy of the device if you’re only testing it on two people,” said Sjoding.
More specific FDA guidance on oximeters is warranted, said Ashraf Fawzy, a professor of medicine at Johns Hopkins University and lead author of a May study published in JAMA Internal Medicine that found providers were more likely to underestimate the level of disease severity and delay treatment for Black and Hispanic Covid-19 patients.
Quicker action from the agency would have been helpful, Fawzy added. The FDA should consider adding a warning label on the devices, said Kimani Toussaint, a professor of engineering at Brown University who is working on potential fixes.
Some experts, like Blackstock, argue the oximeters should be pulled off the market. Others, such as Mohamed, say there needs to be significantly more research before any conclusions are drawn.
“If we are concerned about someone’s breathing or someone’s oxygen level, it’s not safe to completely rely on a pulse oximeter and we always need to confirm it,” Mohamed said.
And fixing the issue in the devices themselves could be a heavy lift.
The FDA spokesperson said it is seeking to broaden the available data on the problem by
funding a prospective clinical trial to inform any recommendation changes. It hopes that research can sort out “sometimes conflicting data that includes non-public information” that manufacturers have provided.
Meanwhile, researchers at Brown University are working on using a single wavelength of light to bypass the skin. That research on healthy patients is in the early stages and saw similar results to commercial devices. The inaccuracies tend to be more prevalent in sicker patients, said researcher Rutendo Jakachira, who works with Toussaint.
Scientists are also looking at using sound as a potential replacement for light as a new method of reading blood oxygen levels.
Clinical trial diversity
Meanwhile, with technology playing an ever-increasing role in health care, experts say that clinical trials, in which people of color have long been underrepresented, need an overhaul.
Lawmakers are aware of the issue. The House last month passed FDA medical product user fee legislation that included language aimed at bolstering clinical trial diversity.
Adrian Aguilera, the head of the Digital Health Equity and Access Lab at the University of California, Berkeley, said that absent diverse participants, the trial outcomes won’t necessarily reflect what will play out in the real world.
Trials are traditionally conducted in person, necessitating that participants come on-site, which can create barriers for low-income people or those with inflexible jobs. Advocates want to use telehealth to draw in a wider range of participants.
Companies should avoid “helicopter” research and instead take time to build relationships with community organizations and people on the ground, Aguilera said.
“What this pulse oximeter situation exemplifies is that if you’re not thinking about bias and racism from the beginning, and you’re not intentional about it, it’s going to be embedded in the technology,” Blackstock said.